Scientists Uncover a Hidden Function of a Cellular Complex: How It Transports Lipids
New research has revealed that the protein channel Sec61/TRAP, best known as the gateway for proteins entering the endoplasmic reticulum — the cell’s protein production factory — also plays a crucial role in transporting lipids across cellular membranes. This surprising discovery highlights the remarkable flexibility with which cells repurpose molecular tools to maintain membrane stability and functionality.
A team of researchers from the J. Heyrovský Institute of Physical Chemistry, Masaryk University, and universities in Tampere and Helsinki, Finland, has uncovered a new function of one of the most important protein channels in the human body — the Sec61/TRAP complex. Previously, it was primarily recognized for enabling newly synthesized proteins to enter the endoplasmic reticulum (ER), the cell’s internal factory where essential proteins and lipids are produced and modified. As shown in a recent article published in the prestigious Journal of the American Chemical Society, the Sec61/TRAP complex has an unexpected additional role: facilitating lipid transport across cellular membranes.
Cell membranes are not passive barriers but dynamic structures composed of lipid bilayers that regulate what enters and exits the cell. To function properly, membranes require a balanced composition on both sides. However, this balance is not automatic — most lipids are synthesized on one side of the membrane and must be efficiently transferred to the other. This task falls to specialized proteins that mediate energy-independent lipid transport.
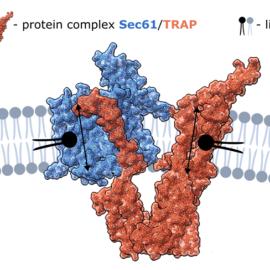
Sec61/TRAP has long been described as a molecular complex responsible for protein translocation across the ER membrane. However, the new study shows that Sec61/TRAP can also transport lipids. Crucially, even when the main Sec61 channel is blocked, lipid translocation continues — suggesting the existence of an alternative pathway. This pathway is provided by the TRAP protein, which forms a so-called polar groove — a narrow cavity through which lipid molecules can slide in a manner reminiscent of swiping a credit card. The polar head of the lipid moves through the groove, while the hydrophobic tail remains embedded in the membrane, minimizing energy consumption.
“The research on Sec61/TRAP builds on earlier work by Finnish scientists who resolved the structure of the complex using electron microscopy. Based on their structural model, a new function of the Sec61/TRAP complex was predicted using computer simulations. At the J. Heyrovský Institute, we then used fluorescence spectroscopy to confirm Sec61/TRAP activity experimentally,” explains Radek Šachl from the J. Heyrovský Institute of Physical Chemistry.
Combining computational and experimental approaches enabled the team to uncover the molecular mechanism by which Sec61/TRAP transports lipids. Owing to its unique structural features, the protein complex enables spontaneous, energy-independent lipid transfer.
“It was like discovering a secret door that had always been right next to the main one,” comment the study’s lead authors. The finding provides new insight into how cells maintain membrane homeostasis. “Initially, it seemed that lipid transport was mediated through the protein channel formed by Sec61. However, after chemically blocking Sec61 with specific inhibitors, we observed that lipid transport persisted at unchanged levels. In line with molecular dynamic simulations, we thus identified a new polar channel, formed by the TRAP domain, that enables lipid transport,” says Jan Šimek from the J. Heyrovský Institute.
The scientific community has previously considered targeting Sec61 to suppress tumor cell growth or halt viral infections. A more accurate understanding of its function provides important context not only for studying membrane physiology but also for the development of new therapeutics.