Group of Molecular Electrochemistry
Current research topics
Current research topics
Single-molecule conductance
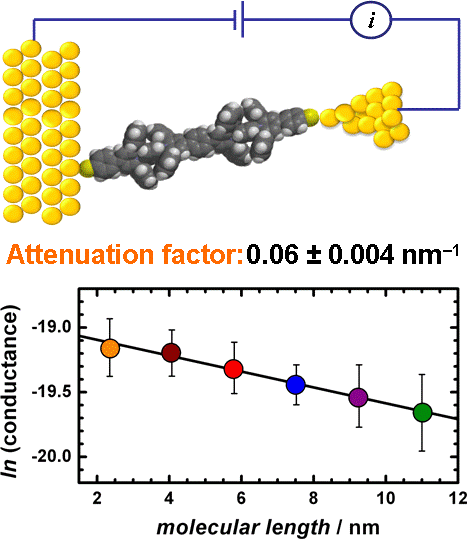 Single-molecule conductance in a series of extended viologen molecules was
measured at room temperature using a gold-molecule-gold scanning tunneling microscopy
break junction arrangement. Conductance values for individual molecules change from 4.8 ± 1.2
nS for the shortest compound to 2.9 ± 1.0 nS for the compound with six repeating units and
length of 11 nm. The latter value is almost 3 orders of magnitude higher than that reported for
all-carbon-based aromatic molecular wires of comparable length. On the basis of the length of
the molecules, an attenuation factor of only 0.06 ± 0.004 nm-1 (0.006 ± 0.0004 Ĺ-1) was
obtained. To the best of our knowledge, this is the smallest value reported for the conductance
attenuation in a series of molecular wires.
Single-molecule conductance in a series of extended viologen molecules was
measured at room temperature using a gold-molecule-gold scanning tunneling microscopy
break junction arrangement. Conductance values for individual molecules change from 4.8 ± 1.2
nS for the shortest compound to 2.9 ± 1.0 nS for the compound with six repeating units and
length of 11 nm. The latter value is almost 3 orders of magnitude higher than that reported for
all-carbon-based aromatic molecular wires of comparable length. On the basis of the length of
the molecules, an attenuation factor of only 0.06 ± 0.004 nm-1 (0.006 ± 0.0004 Ĺ-1) was
obtained. To the best of our knowledge, this is the smallest value reported for the conductance
attenuation in a series of molecular wires.
Oscillations of extended viologen
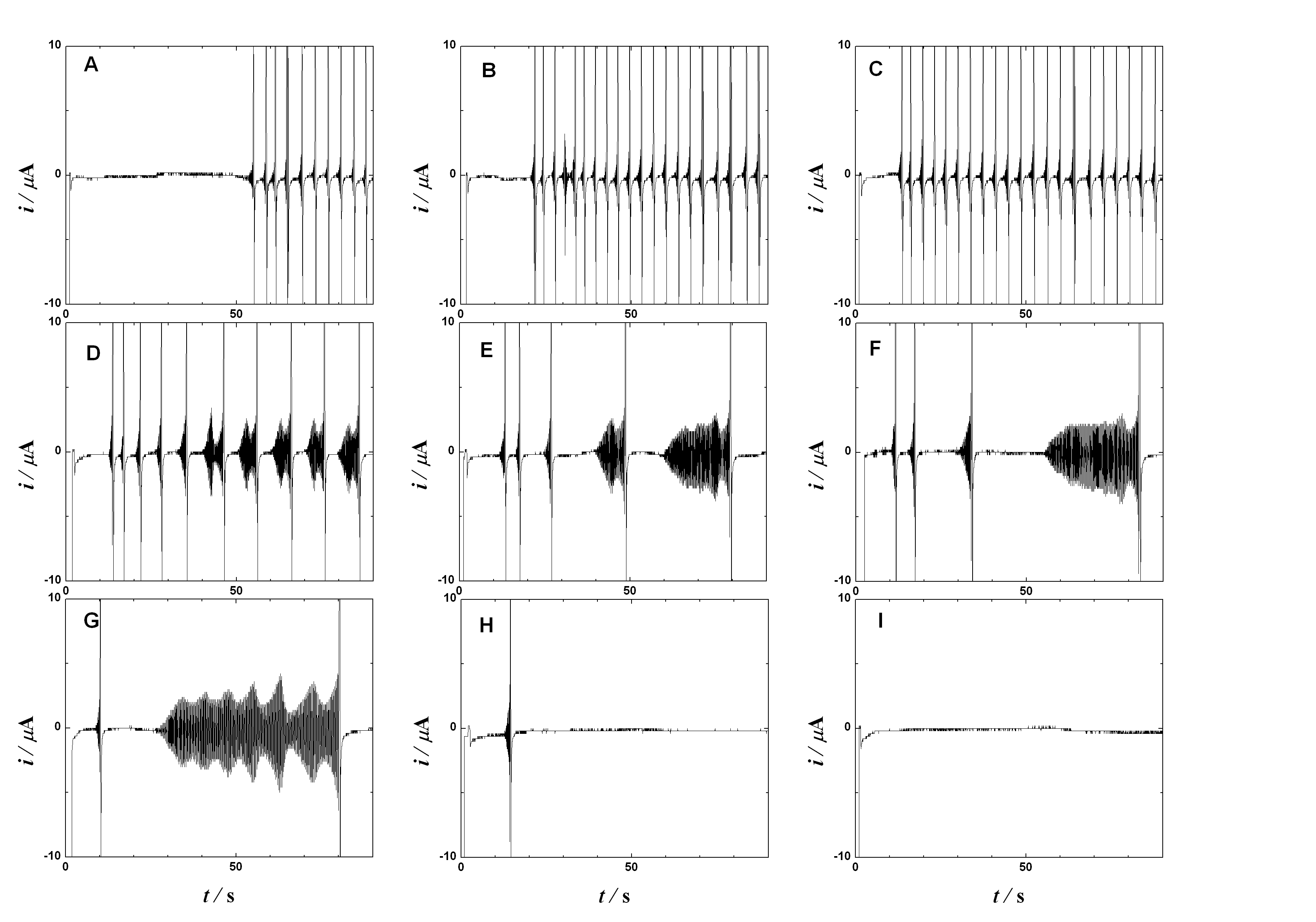
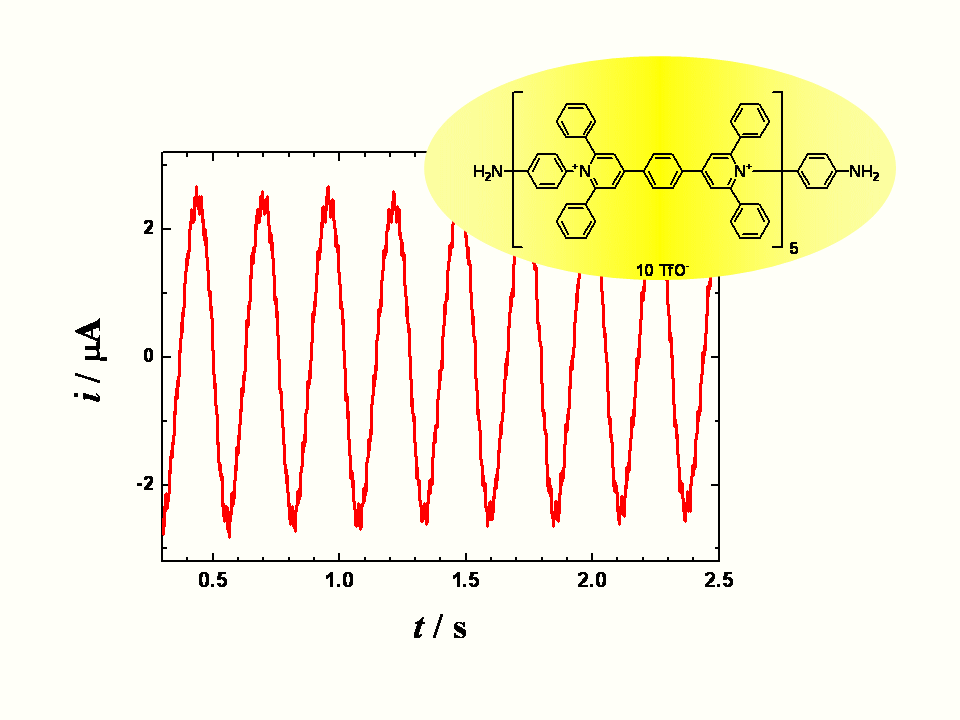 A long organic molecule with five bipyridinium functions separated by benzene rings
(extended viologen) undergoes a reversible multi-step electron transfer. Here we show
that this decacation accepts electrons at the heterogeneous interface with the occurrence
of the periodically changing electric reduction currents. According to the applied bias
voltage the observed current time dependence changes from chaotic through periodic and
irregular to sinusoidal and finally to monotonous. A careful choice of the controlling
parameters yields the sustained periodic sinusoidal currents lasting for a prolonged time.
Oscillations stem from a mutual interplay of the heterogeneous supply of electrons and
the homogeneous redox reactions (disproportionation) between the transient redox forms.
In difference to many other electrochemical oscillating systems the described
oscillations do not require any additional external impedance. The principle
of these oscillatory currents may serve as a model of a truly ‘molecular oscillator’.
A long organic molecule with five bipyridinium functions separated by benzene rings
(extended viologen) undergoes a reversible multi-step electron transfer. Here we show
that this decacation accepts electrons at the heterogeneous interface with the occurrence
of the periodically changing electric reduction currents. According to the applied bias
voltage the observed current time dependence changes from chaotic through periodic and
irregular to sinusoidal and finally to monotonous. A careful choice of the controlling
parameters yields the sustained periodic sinusoidal currents lasting for a prolonged time.
Oscillations stem from a mutual interplay of the heterogeneous supply of electrons and
the homogeneous redox reactions (disproportionation) between the transient redox forms.
In difference to many other electrochemical oscillating systems the described
oscillations do not require any additional external impedance. The principle
of these oscillatory currents may serve as a model of a truly ‘molecular oscillator’.
Electrochemical nitrogen fixation
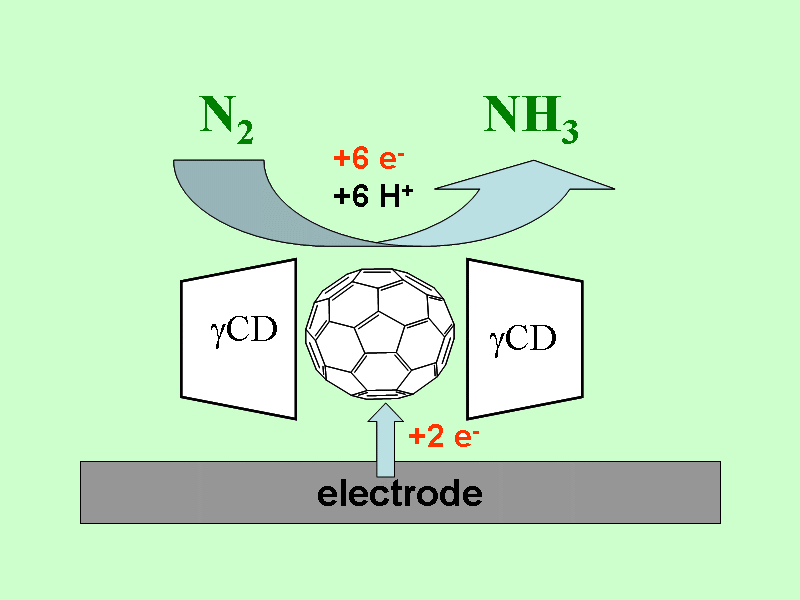 We found an electrochemical conversion of N2 to NH3 at ambient pressure
and 60o C, which is mediated by reduced C60 inside the molecular
cavity of gamma-cyclodextrin. The formation of ammonia was confirmed by photo-acoustic spectroscopy.
The absence of hydrazine formation was proven.
We found an electrochemical conversion of N2 to NH3 at ambient pressure
and 60o C, which is mediated by reduced C60 inside the molecular
cavity of gamma-cyclodextrin. The formation of ammonia was confirmed by photo-acoustic spectroscopy.
The absence of hydrazine formation was proven.
Transient temperature of adsorbed layers of pesticides
correlates with contamination
Two pesticides, atrazine and terbutylazine, have very similar chemical structures differing
only by iso-propyl and tert-butyl substituents on their 6-amino-groups. This minor structural
difference causes profound effects in decomposition rates in the environment leading to a ban
of atrazine in the European Union. W published a study of adsorption at ideally polarized
electrochemical interface in the absence of specifically adsorbed halides. The interfacial
charge and the temperature determine which type of an adsorbed film is formed. The double
layer capacitance measurements yield the critical temperature of the surface film transition,
which is markedly different for the two pesticides. The time-resolved impedance spectroscopy
indicates slow changes within the film structure that becomes disordered and can be
characterized in terms of the fractal geometry.
Reduction of nitro-aromatic radical anions
is strongly influenced by interfacial charge and other interactions
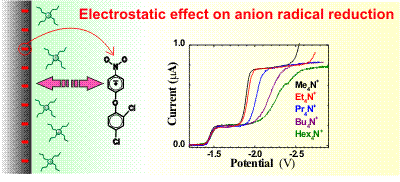 Anion radicals of bifenox and also of nitrobenzene are irreversibly reduced in acetonitrile in a three
electron process which is strongly dependent on the concentration and cation size of the indifferent
electrolyte. The lower is the concentration of the indifferent electrolyte the slower is the electron
transfer rate. The correction of the apparent rate constants for the influence of the Phi-2 potential
at the outer Helmholtz plane (the Frumkin correction) holds for the case of tetramethylammonium
hexafluorophosphate and is consistent with the second electron transfer being the rate determining step.
The electron transfer rates corrected for the value of the 2 potential are still different
for larger cations and decrease in a series tetramethyl- > tetraethyl- > tetrapropyl- >
tetrabutyl- > tetrahexylammonuim. Corrected rate constants decay exponentially with the
number of carbon bonds of the indifferent cation with an exponent beta = 0.83. Results show
that a seemingly established reaction kinetics of aromatic nitro compounds is quite
complex in many aspects.
Anion radicals of bifenox and also of nitrobenzene are irreversibly reduced in acetonitrile in a three
electron process which is strongly dependent on the concentration and cation size of the indifferent
electrolyte. The lower is the concentration of the indifferent electrolyte the slower is the electron
transfer rate. The correction of the apparent rate constants for the influence of the Phi-2 potential
at the outer Helmholtz plane (the Frumkin correction) holds for the case of tetramethylammonium
hexafluorophosphate and is consistent with the second electron transfer being the rate determining step.
The electron transfer rates corrected for the value of the 2 potential are still different
for larger cations and decrease in a series tetramethyl- > tetraethyl- > tetrapropyl- >
tetrabutyl- > tetrahexylammonuim. Corrected rate constants decay exponentially with the
number of carbon bonds of the indifferent cation with an exponent beta = 0.83. Results show
that a seemingly established reaction kinetics of aromatic nitro compounds is quite
complex in many aspects.
Copyright 2007 J. Heyrovský Institute of Physical Chemistry, v.v.i.,
Design by L. P.
 Single-molecule conductance in a series of extended viologen molecules was
measured at room temperature using a gold-molecule-gold scanning tunneling microscopy
break junction arrangement. Conductance values for individual molecules change from 4.8 ± 1.2
nS for the shortest compound to 2.9 ± 1.0 nS for the compound with six repeating units and
length of 11 nm. The latter value is almost 3 orders of magnitude higher than that reported for
all-carbon-based aromatic molecular wires of comparable length. On the basis of the length of
the molecules, an attenuation factor of only 0.06 ± 0.004 nm-1 (0.006 ± 0.0004 Ĺ-1) was
obtained. To the best of our knowledge, this is the smallest value reported for the conductance
attenuation in a series of molecular wires.
Single-molecule conductance in a series of extended viologen molecules was
measured at room temperature using a gold-molecule-gold scanning tunneling microscopy
break junction arrangement. Conductance values for individual molecules change from 4.8 ± 1.2
nS for the shortest compound to 2.9 ± 1.0 nS for the compound with six repeating units and
length of 11 nm. The latter value is almost 3 orders of magnitude higher than that reported for
all-carbon-based aromatic molecular wires of comparable length. On the basis of the length of
the molecules, an attenuation factor of only 0.06 ± 0.004 nm-1 (0.006 ± 0.0004 Ĺ-1) was
obtained. To the best of our knowledge, this is the smallest value reported for the conductance
attenuation in a series of molecular wires. Oscillations of extended viologen
 A long organic molecule with five bipyridinium functions separated by benzene rings
(extended viologen) undergoes a reversible multi-step electron transfer. Here we show
that this decacation accepts electrons at the heterogeneous interface with the occurrence
of the periodically changing electric reduction currents. According to the applied bias
voltage the observed current time dependence changes from chaotic through periodic and
irregular to sinusoidal and finally to monotonous. A careful choice of the controlling
parameters yields the sustained periodic sinusoidal currents lasting for a prolonged time.
Oscillations stem from a mutual interplay of the heterogeneous supply of electrons and
the homogeneous redox reactions (disproportionation) between the transient redox forms.
In difference to many other electrochemical oscillating systems the described
oscillations do not require any additional external impedance. The principle
of these oscillatory currents may serve as a model of a truly ‘molecular oscillator’.
A long organic molecule with five bipyridinium functions separated by benzene rings
(extended viologen) undergoes a reversible multi-step electron transfer. Here we show
that this decacation accepts electrons at the heterogeneous interface with the occurrence
of the periodically changing electric reduction currents. According to the applied bias
voltage the observed current time dependence changes from chaotic through periodic and
irregular to sinusoidal and finally to monotonous. A careful choice of the controlling
parameters yields the sustained periodic sinusoidal currents lasting for a prolonged time.
Oscillations stem from a mutual interplay of the heterogeneous supply of electrons and
the homogeneous redox reactions (disproportionation) between the transient redox forms.
In difference to many other electrochemical oscillating systems the described
oscillations do not require any additional external impedance. The principle
of these oscillatory currents may serve as a model of a truly ‘molecular oscillator’.

Electrochemical nitrogen fixation
 We found an electrochemical conversion of N2 to NH3 at ambient pressure
and 60o C, which is mediated by reduced C60 inside the molecular
cavity of gamma-cyclodextrin. The formation of ammonia was confirmed by photo-acoustic spectroscopy.
The absence of hydrazine formation was proven.
We found an electrochemical conversion of N2 to NH3 at ambient pressure
and 60o C, which is mediated by reduced C60 inside the molecular
cavity of gamma-cyclodextrin. The formation of ammonia was confirmed by photo-acoustic spectroscopy.
The absence of hydrazine formation was proven.
Transient temperature of adsorbed layers of pesticides
correlates with contamination
Two pesticides, atrazine and terbutylazine, have very similar chemical structures differing
only by iso-propyl and tert-butyl substituents on their 6-amino-groups. This minor structural
difference causes profound effects in decomposition rates in the environment leading to a ban
of atrazine in the European Union. W published a study of adsorption at ideally polarized
electrochemical interface in the absence of specifically adsorbed halides. The interfacial
charge and the temperature determine which type of an adsorbed film is formed. The double
layer capacitance measurements yield the critical temperature of the surface film transition,
which is markedly different for the two pesticides. The time-resolved impedance spectroscopy
indicates slow changes within the film structure that becomes disordered and can be
characterized in terms of the fractal geometry.
Reduction of nitro-aromatic radical anions
is strongly influenced by interfacial charge and other interactions
 Anion radicals of bifenox and also of nitrobenzene are irreversibly reduced in acetonitrile in a three
electron process which is strongly dependent on the concentration and cation size of the indifferent
electrolyte. The lower is the concentration of the indifferent electrolyte the slower is the electron
transfer rate. The correction of the apparent rate constants for the influence of the Phi-2 potential
at the outer Helmholtz plane (the Frumkin correction) holds for the case of tetramethylammonium
hexafluorophosphate and is consistent with the second electron transfer being the rate determining step.
The electron transfer rates corrected for the value of the 2 potential are still different
for larger cations and decrease in a series tetramethyl- > tetraethyl- > tetrapropyl- >
tetrabutyl- > tetrahexylammonuim. Corrected rate constants decay exponentially with the
number of carbon bonds of the indifferent cation with an exponent beta = 0.83. Results show
that a seemingly established reaction kinetics of aromatic nitro compounds is quite
complex in many aspects.
Anion radicals of bifenox and also of nitrobenzene are irreversibly reduced in acetonitrile in a three
electron process which is strongly dependent on the concentration and cation size of the indifferent
electrolyte. The lower is the concentration of the indifferent electrolyte the slower is the electron
transfer rate. The correction of the apparent rate constants for the influence of the Phi-2 potential
at the outer Helmholtz plane (the Frumkin correction) holds for the case of tetramethylammonium
hexafluorophosphate and is consistent with the second electron transfer being the rate determining step.
The electron transfer rates corrected for the value of the 2 potential are still different
for larger cations and decrease in a series tetramethyl- > tetraethyl- > tetrapropyl- >
tetrabutyl- > tetrahexylammonuim. Corrected rate constants decay exponentially with the
number of carbon bonds of the indifferent cation with an exponent beta = 0.83. Results show
that a seemingly established reaction kinetics of aromatic nitro compounds is quite
complex in many aspects.
Copyright 2007 J. Heyrovský Institute of Physical Chemistry, v.v.i.,
Design by L. P.
 A long organic molecule with five bipyridinium functions separated by benzene rings
(extended viologen) undergoes a reversible multi-step electron transfer. Here we show
that this decacation accepts electrons at the heterogeneous interface with the occurrence
of the periodically changing electric reduction currents. According to the applied bias
voltage the observed current time dependence changes from chaotic through periodic and
irregular to sinusoidal and finally to monotonous. A careful choice of the controlling
parameters yields the sustained periodic sinusoidal currents lasting for a prolonged time.
Oscillations stem from a mutual interplay of the heterogeneous supply of electrons and
the homogeneous redox reactions (disproportionation) between the transient redox forms.
In difference to many other electrochemical oscillating systems the described
oscillations do not require any additional external impedance. The principle
of these oscillatory currents may serve as a model of a truly ‘molecular oscillator’.
A long organic molecule with five bipyridinium functions separated by benzene rings
(extended viologen) undergoes a reversible multi-step electron transfer. Here we show
that this decacation accepts electrons at the heterogeneous interface with the occurrence
of the periodically changing electric reduction currents. According to the applied bias
voltage the observed current time dependence changes from chaotic through periodic and
irregular to sinusoidal and finally to monotonous. A careful choice of the controlling
parameters yields the sustained periodic sinusoidal currents lasting for a prolonged time.
Oscillations stem from a mutual interplay of the heterogeneous supply of electrons and
the homogeneous redox reactions (disproportionation) between the transient redox forms.
In difference to many other electrochemical oscillating systems the described
oscillations do not require any additional external impedance. The principle
of these oscillatory currents may serve as a model of a truly ‘molecular oscillator’.
Electrochemical nitrogen fixation
 We found an electrochemical conversion of N2 to NH3 at ambient pressure
and 60o C, which is mediated by reduced C60 inside the molecular
cavity of gamma-cyclodextrin. The formation of ammonia was confirmed by photo-acoustic spectroscopy.
The absence of hydrazine formation was proven.
We found an electrochemical conversion of N2 to NH3 at ambient pressure
and 60o C, which is mediated by reduced C60 inside the molecular
cavity of gamma-cyclodextrin. The formation of ammonia was confirmed by photo-acoustic spectroscopy.
The absence of hydrazine formation was proven.
Transient temperature of adsorbed layers of pesticides
correlates with contamination
Two pesticides, atrazine and terbutylazine, have very similar chemical structures differing
only by iso-propyl and tert-butyl substituents on their 6-amino-groups. This minor structural
difference causes profound effects in decomposition rates in the environment leading to a ban
of atrazine in the European Union. W published a study of adsorption at ideally polarized
electrochemical interface in the absence of specifically adsorbed halides. The interfacial
charge and the temperature determine which type of an adsorbed film is formed. The double
layer capacitance measurements yield the critical temperature of the surface film transition,
which is markedly different for the two pesticides. The time-resolved impedance spectroscopy
indicates slow changes within the film structure that becomes disordered and can be
characterized in terms of the fractal geometry.
Reduction of nitro-aromatic radical anions
is strongly influenced by interfacial charge and other interactions
 Anion radicals of bifenox and also of nitrobenzene are irreversibly reduced in acetonitrile in a three
electron process which is strongly dependent on the concentration and cation size of the indifferent
electrolyte. The lower is the concentration of the indifferent electrolyte the slower is the electron
transfer rate. The correction of the apparent rate constants for the influence of the Phi-2 potential
at the outer Helmholtz plane (the Frumkin correction) holds for the case of tetramethylammonium
hexafluorophosphate and is consistent with the second electron transfer being the rate determining step.
The electron transfer rates corrected for the value of the 2 potential are still different
for larger cations and decrease in a series tetramethyl- > tetraethyl- > tetrapropyl- >
tetrabutyl- > tetrahexylammonuim. Corrected rate constants decay exponentially with the
number of carbon bonds of the indifferent cation with an exponent beta = 0.83. Results show
that a seemingly established reaction kinetics of aromatic nitro compounds is quite
complex in many aspects.
Anion radicals of bifenox and also of nitrobenzene are irreversibly reduced in acetonitrile in a three
electron process which is strongly dependent on the concentration and cation size of the indifferent
electrolyte. The lower is the concentration of the indifferent electrolyte the slower is the electron
transfer rate. The correction of the apparent rate constants for the influence of the Phi-2 potential
at the outer Helmholtz plane (the Frumkin correction) holds for the case of tetramethylammonium
hexafluorophosphate and is consistent with the second electron transfer being the rate determining step.
The electron transfer rates corrected for the value of the 2 potential are still different
for larger cations and decrease in a series tetramethyl- > tetraethyl- > tetrapropyl- >
tetrabutyl- > tetrahexylammonuim. Corrected rate constants decay exponentially with the
number of carbon bonds of the indifferent cation with an exponent beta = 0.83. Results show
that a seemingly established reaction kinetics of aromatic nitro compounds is quite
complex in many aspects.
 We found an electrochemical conversion of N2 to NH3 at ambient pressure
and 60o C, which is mediated by reduced C60 inside the molecular
cavity of gamma-cyclodextrin. The formation of ammonia was confirmed by photo-acoustic spectroscopy.
The absence of hydrazine formation was proven.
We found an electrochemical conversion of N2 to NH3 at ambient pressure
and 60o C, which is mediated by reduced C60 inside the molecular
cavity of gamma-cyclodextrin. The formation of ammonia was confirmed by photo-acoustic spectroscopy.
The absence of hydrazine formation was proven. Transient temperature of adsorbed layers of pesticides
correlates with contamination
Reduction of nitro-aromatic radical anions
is strongly influenced by interfacial charge and other interactions
 Anion radicals of bifenox and also of nitrobenzene are irreversibly reduced in acetonitrile in a three
electron process which is strongly dependent on the concentration and cation size of the indifferent
electrolyte. The lower is the concentration of the indifferent electrolyte the slower is the electron
transfer rate. The correction of the apparent rate constants for the influence of the Phi-2 potential
at the outer Helmholtz plane (the Frumkin correction) holds for the case of tetramethylammonium
hexafluorophosphate and is consistent with the second electron transfer being the rate determining step.
The electron transfer rates corrected for the value of the 2 potential are still different
for larger cations and decrease in a series tetramethyl- > tetraethyl- > tetrapropyl- >
tetrabutyl- > tetrahexylammonuim. Corrected rate constants decay exponentially with the
number of carbon bonds of the indifferent cation with an exponent beta = 0.83. Results show
that a seemingly established reaction kinetics of aromatic nitro compounds is quite
complex in many aspects.
Anion radicals of bifenox and also of nitrobenzene are irreversibly reduced in acetonitrile in a three
electron process which is strongly dependent on the concentration and cation size of the indifferent
electrolyte. The lower is the concentration of the indifferent electrolyte the slower is the electron
transfer rate. The correction of the apparent rate constants for the influence of the Phi-2 potential
at the outer Helmholtz plane (the Frumkin correction) holds for the case of tetramethylammonium
hexafluorophosphate and is consistent with the second electron transfer being the rate determining step.
The electron transfer rates corrected for the value of the 2 potential are still different
for larger cations and decrease in a series tetramethyl- > tetraethyl- > tetrapropyl- >
tetrabutyl- > tetrahexylammonuim. Corrected rate constants decay exponentially with the
number of carbon bonds of the indifferent cation with an exponent beta = 0.83. Results show
that a seemingly established reaction kinetics of aromatic nitro compounds is quite
complex in many aspects.