Yes Arrhenius, electrolytes are indeed partially dissociated in
solutions at all concentrations
*R. Heyrovská
Symposium Svante
Arrhenius

November 27 - 29, 2003, Uppsala, Sweden, commemorating
the Centenary of
the award of the
nobel prize to
svante arrhenius
*(J. Heyrovský Inst. of Phys. Chem., Academy of Sciences of
the Czech Republic, Dolejškova 3, 182 23 Prague 8, Czech Republic.)
(photo: dbhs.wvusd.k12.ca.us/Gallery)
Contents
I: The theory of electrolytic dissociation due to Arrhenius, (p.3).
II: The anomaly of strong electrolytes, (p.4).
III:
The empirical theory of electrolytes, (p.5);
Equations for
properties of strong electrolytes based on complete dissociation, e.g.,
NaCl ¢ Na+ + Cl-, (p.6).
IV.1: Arrhenius‘
theory of partial dissociation found valid from “0 to 3m“, (p.7).
IV.2: Arrhenius‘
theory of partial dissociation found valid from “0 to saturation“,
(p.9);
Equations for
properties of strong electrolytes based on partial dissociation, e.g.,
NaCl D Na+ + Cl-, (p.10).
IV.3: Guldberg and Waage’s law found valid for 1:1
strong electrolytes, (p.12).
IV.4: Bjerrum’s theory found valid for 1:1 strong
electrolytes, (p.13).
V:
Conclusion, (p.14).
References,
(pp. 15 – 26).
Figures (9)
and Tables (3)
Photos, I:
Arrhenius in Spitzbergen in 1896 and II: the same place, “Virgohamn“, in 1967
(photo by R. Heyrovská).
I: The theory of
electrolytic dissociation due to ARRHENIUS
ARRHENIUS
[1] made a major contribution to solution science when he discovered in 1883
that an electrolyte like NaCl dissociates in water partly into the “active
(ions) form, the rest being the inactive (undissociated)“ form:
(1 - a)c
NaCl D (ac) Na+ + (ac) Cl-
where a £ 1 is
the degree of dissociation at concentration c. Arrhenius calculated a as
the ratio, L/Lo, where L is
the equivalent conductivity of the solution at concentration c and Lo is
that at infinite dilution, when a = 1.
The total number of moles of solute in the solution per mole of the dissolved
electrolyte, is given by,
i = (1 - a) + 2a = (1
+ a) £ 2
This factor, i,
appeared in van’t Hoff‘s [2] equation for the osmotic pressure, pos
posV =
iRT
where V is the
molar volume.
Ostwald [3] found that Arrhenius‘ theory confirmed Guldberg and Waage’s [4a,b] law for the
dissociation constant K of a weak acid:
K = a2c/(1
- a) = (L/Lo)2c/[1
- (L/Lo)]
The above
successes brought Arrhenius,
van’t Hoff and Ostwald, together as an “Ionist Trio“, who foresaw the great
potentialities of the new theory. Ostwald’s laboratories soon became the
“learning center“ for scientists from far and wide.
II. The anomaly of strong
electrolytes
Subsequently, it was found that the use of
the conductivity ratio for a was
satisfactory only for dilute solutions, especially for highly dissociated
electrolytes, a typical example being NaCl in aqueous solutions.
Attempts were made at modifying the conductivity ratio and taking hydration of
the solute into account.
Bousfield [5] showed, although
approximately, with the degrees of dissociation evaluated from freezing point
depressions, that Raoult’s [6] law for the vapour pressure of solutions of
non-electrolytes, is also valid for electrolytes, on allowing for their partial
dissociation and hydration.
III. The empirical theory of
electrolytes
“In the absence of any idea as to
the concentration of the undissociated electrolyte“, Lewis and Randall (L &
R) [7] proposed an empirical dissociation constant,
Ka = a+a-/aB
= 1
where a+
= a- = aB1/2 = a± = mg± is the mean
molal ionic activity in a solution of molal concentration m (m moles of solute
per kg of solvent), aB is the molal activity of the undissociated
electrolyte and g± is
the mean molal ionic activity coefficient.
The observed linear dependence
(approximate) of lng± and
other solution properties on √m or √c for “very dilute solutions“,
was explained by Debye and Hückel (D & H) [8a] as due to interionic
interactions, by assuming complete dissociation of the electrolyte,
NaCl ¢ Na+
+ Cl-
The success of the D & H equations
(which are for complete dissociation of the electrolyte) was taken as an
endorsement of L & R’s definition of g±,
although it involves the activity of the “undissociated“ electrolyte. L
& R [7] stated that for (their) thermodynamics, it did not matter whether
one considers the electrolyte as partially or completely dissociated.
The D & H equations were subsequently
extended, by adding more parameters and terms to fit the data for higher and
higher concentrations.
Bjerrum [9] developed a theory of ionic
association, but he too considered that ion pairs were unlikely in solutions of
1:1 electrolytes like NaCl(aq).
Equations for properties of strong electrolytes based
on complete dissociation, NaCl ¢ Na+
+ Cl-
A: Equivalent conductivity, L: (0 ~ 3m), [10]
Lo ‑ L » (B1Lo + B2)Öc c < 0.1m.
L =
(h/hA)[ Lo ‑
B2Öc/(1+
BaÖc)][1‑
B1ÖcF/(1+
BaÖc)]
B: Diffusion coefficient, D: (0 ~ 3m), [10]
D = (Do+D1+D2){1+0.036m[(D*/Do)
- nh]} (1+mdlng±/dm)(hA/h)
C: Solvent activity, aA = (pA/pAo):
(0 ~ 6m), [11]
= exp(-2mf/55.51);
f : osmotic coefficient
(non-ideality coefficient), (subscript A is for solvent)
posVA = -RTln aA
= -RT(2mf/55.51)
f
= 1- zMzXAfI1/2/(1+bI1/2) + m(2nMnX/n)(bMX(o) +bMX(1)exp(- aI1/2)
+ m2(4nM2nXzM /n)CMX
D: E.M.F. of concentration cells, DE: (0.01 ~ 6m), [11]
[= -(2RT/F)ln(mg±)];
g±: mean ionic activity
coefficient (non ideality factor)
ln(g±) = - zMzXAf [(I1/2/(1+bI1/2)+(2/b)ln(1+bI1/2)]
+ m(2nMnX/n){2bMX(o)+(2bMX(1)/a2I)[1-(1+aI1/2-a2I/2) exp(- aI1/2)]}
+ m2(2nM2nX zM/n)(3CMX)
E: Molal volumes, Vm: (0 ~
saturation), [12]
Vf,MX = (Vm - VAo)/m
= VMXo+Ao+A1bMX(o)V+A2bMX(1)V+A3bMX(2)V+A4CMXV
IV.1: Arrhenius‘
theory of partial dissociation found valid from “0 to 3m“
The author realized that the above
theories had amounted to converting experimental data into ²catalogues² of
best-fitting parameters, rather than “explaining“ the significance of the
observed results. See [13] for similar opinions. Therefore, the author
preferred to re-analyse the available data by a careful and systematic
investigation (1980 -). It became gradually evident by 1984 that Arrhenius‘ idea
of partial dissociation was indeed correct. The author obtained
experimental support for the presence of ion pairs in the work (in 1992) on
X-ray diffraction studies of saturated alkali halide solutions, by Ohtaki and
Fukushima [14].
Presented below are the main points of the
author’s quantitative re-establishment of the theory of partial dissociation.
Details can be found in articles [15] – [62].
By systematic analyses of the existing
experimental data, the author found [15], [16] that van’t Hoff’s gas-solution
analogy was valid (for higher pressures of gases and) higher concentrations of
solutions, with the van’t Hoff‘s factor i in Bousfield’s equation.
Subsequently, [17] – [24], with the
degrees of dissociation (a) and
hydration numbers (constant, independent of m) evaluated from osmotic pressure
(os.p.) using osmotic coefficient (f) data, the solution properties could be explained over
a large concentration range, (0 to 3m), see Figs. 1 & 2.
The Debye, Hückel and Onsager’s (D, H
& O) [8a, b] „√c“
law for conductivity, explaining Kohlrausch’s [8c] observation, was found to be
an “asymptotic limiting law“ for complete dissociation at infinite
dilution. For dilute solutions, L vs (1 - a) gave a
linear dependence over a larger range of concentrations (0 - 0.1m) than the (D, H
& O) law, e.g., see Fig. 1a.
The quantitative
correlations were further improved [25] – [39] by evaluating a and hydration numbers (ns)
from the equation for vapour pressure using the data on osmotic coefficients (f).
IV.2: Arrhenius‘
theory of partial dissociation found valid from “0 to saturation“
A re-examination of the results obtained
so far, showed that the degrees of dissociation a obtained from osmotic pressure (os.p.) were nearly the
same as that evaluated from vapour pressure (v.p.). On using the a values evaluated from v.p. into the
equation for os.p., the latter (a bulk property) gave a lower hydration number
than the former (an interfacial property) over the entire concentration range
from “0 to saturation“.
This finding enabled the author to
evaluate values of a and
hydration numbers (from the existing data on osmotic coefficients), which
explained quantitatively the basic thermodynamic properties of NaCl(aq)
from “zero to saturation“, for the first time. See Figs. 3
& 4, Tables 1 & 2 and [40] – [44].
Thus, as the actual molalities of ions
(2am) and ion pairs
[(1 - a)m] became
available since 1996, the non-ideality factors, g± and f (evaluated on the basis of complete
dissociation) became un-necessary. See Fig.
5.
Equations for properties of strong electrolytes based
on partial dissociation,
NaCl D Na+ + Cl-
(A): Equivalent
conductivity (L): [17, 22, 32]
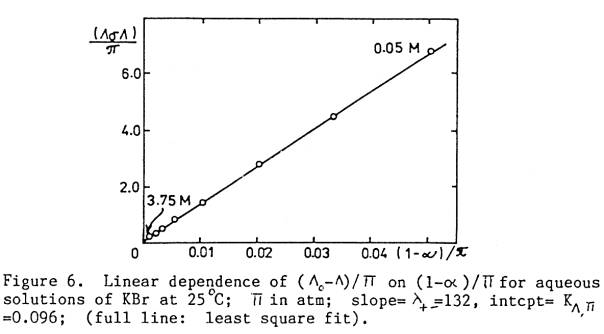
Lo ‑ L » L+‑(1 ‑ a) … (0 to 0.1m)
Lo ‑ L = L+‑(1 ‑ a) + KL,p pos … (0 to ~ 3m)
where L+‑ and KL,p are
constants, obtained as the slope and intercept of the linear plot of (Lo ‑ L)/pos vs (1 ‑ a)/pos. See Figs.
1a,b.
Note: The results have to be extended up to saturation, using the
data in [43], [45], [48].
(B): Diffusion
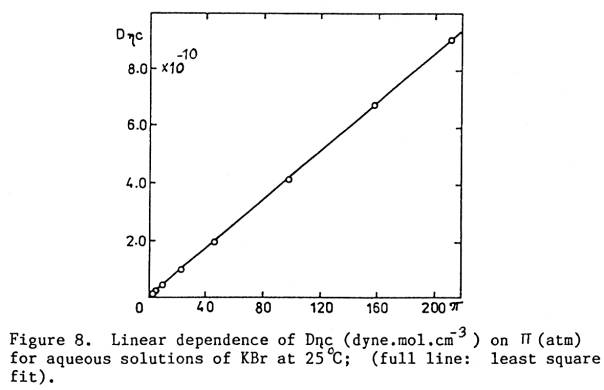
coefficient (D): [18, 32, 35]
D = (pos/c)[1/(hDNAv)] … (0 to ~ 3m)
Do = 2kT/hD
… (at infinite dilution)
where hD is the Stokes factor, NAv is the Avogadro
number. The product Dhc increases linearly with pos. Since NAvD is the slope, one obtains D, for calculating Do. See Fig. 2.
Note: The results have to be extended up to saturation, using the
data in [43], [45], [48].
(C): Solvent
activity, aA = (pA/pAo): [40 -
46, 48]
aA = NAfs
= nAfs/(nAfs+im) [=exp(-2mf/55.51)]
pos = iRT/VAfb =
iRT(55.51m/nAfb)dAfb = 2RTmfdAfb
-aAlnaA/(1-
aA) = nAfs/nAfb
... “0 to
saturation”
where nAfs =
(55.51 - mns), nAfb = (55.51- mnb) are the
molalities of free water, ns and nb are surface and bulk
hydration numbers, VAfb is the volume of free water per mole m, and
dAfb is the density of free
water. The values of ns, nb and i can be obtained by using the above relations
and the available data on aA . See Figs. 3 - 5.
(D): E.M.F. of
concentration cells, DE :
[40 - 46, 48, 55] [DE = -(2RT/F)ln(mg±)]; (DE
from g±, data):
DE
= - dA(2RT/F) ln[(am/nAfs)/rso]
... (~ 0.001 to
saturation)
where nAfs = (55.51
- mns), (am/nAfs) = rs, and dA is the slope of DE
vs ln rs straight line. See Fig. 6.
(E): Molal
volumes, Vm: [43, 48, 53], “0 to saturation”
Vm - VAo
= m[(1- a)VBo+
afvo)]; (m < ma,min)
Vm - VA
= am(fvo + dVd)
= am(VBo+
dVel); (m > ma,min)
where VAo is the volume of 1kg of
water in the pure state; VBo is the volume of one mole of
the electrolyte; fvo = (V++
V- + dVel);
V++ V- is the sum of the volumes per mole of the ions; dVel is the electrostriction, dVd
= VBo- (V++V-) and VA
< VAo. See Fig. 7.
The above interpretation was found to be
valid for many 1:1 strong electrolytes [46], [48] and also monovalent sulphates
[54]; (some up to saturation). The linearity of the graph of surface vs bulk
hydration numbers for the electrolytes in Table 3, is shown in Fig.
8.
IV.3: Guldberg and Waage’s law found valid
for 1:1 strong electrolytes
The finding that the dissociation
constant, Km = a2m/(1
- a) is not a constant, had led L
& R [7] to suggest g±, the
activity coefficient, as a correction factor for m. The author [43], [46], [48]
found that the nonconstancy of Km was due to the use of m as the
unit of concentration. The actual dissociation constant Kd involves
“concentrations“ am/(Vi)soln and (1 - a)m/(Vip)soln,
where (Vi)soln and (Vip)soln are
the volumes of solution occupied by the ions and ions pairs respectively. The
dissociation “constant“, Kd is given by,
Kd = {(am/Vi)2/[(1
- a)m/Vip]}soln
= Kcr
= [Vcr/(V+ + V-)2]cr =
const
where
Vcr and (V++V-) are the volumes per mole of
the crystal and ions respectively. For NaCl (aq) at 25 oC, from „zero
to saturation“, Kd = 0.080 mol.cm-3.
Thus, the dissociation takes place in solution
such that Kd = Kcr = constant, which
demonstrates the beautiful and simple workings of “Nature“ - (Occam’s rule!).
IV.4: Bjerrum’s theory found valid for 1:1
strong electrolytes
Bjerrum [9] thought that the critical
distance (q = 3.57 Å at 25oC) of approach of the oppositely
charged ions in 1:1 electrolytes was too large for ion pair formation. Since
now the degrees of dissociation are known, the author used Bjerrum‘s equation
[9], [12],
(1- a) =
[2.755 f(a)]c
where f(a) is a
function of the mean distance of closest approach, a, of the oppositely charged
ions, to calculate (for the first time) the distance, a, for NaCl(aq) from “zero
to saturation“. The value of a was found to increase from 1.85 Å at
0.1m to 3.53 Å (< q) at saturation. See [47], [51] and Fig. 9.
V: Conclusion
The author
would like to conclude by quoting the words (valid today!) of Dr. H. R.
Törnebladh, President of the Royal Swedish Academy of Sciences,
in his Nobel Prize Presentation Speech (on December 10, 1903):
“Doctor. The world of
science already recognizes the importance and value of your theory, but its
lustre will continue to increase in the days to come, as you yourself and
others use it to advance the science of chemistry.“
References
I –
III:
1. Arrhenius, S.: Z. Physik. Chem. I (1887) 631; Nobel Lecture,
December 11, 1903; J. Amer. Chem. Soc., 34 (1912) 353.
2. van’t Hoff, J. H.: Z. Physik. Chem. I (1887)
481.
3. Ostwald, W.: Z. Physik. Chem. 2 (1888)
270.
4a. Waage, P. and Guldberg, C. M.: Forhandlinger: Videnskabs-Selskabet i Christiana 1864, 35; from: http://chimie.scola.ac-Paris.fr/sitedechimie/hist_chi/text_origin/guldberg_waage/Concerning-Affinity.htm;
see also [4b].
4b. Moelwyn-Hughes, E. A.: “Physical
Chemistry“, Pergamon, London, 1957.
5. Bousfield, W. R.: Trans. Faraday Soc.,
13 (1917) 141.
6. Raoult, F. M.: Z. Physik. Chem. 2 (1888)
353.
7. Lewis, G. N. and Randall, M.: J. Amer. Chem. Soc., 43 (1921) 1112.
8a. Debye, P. and Hückel, E.: Phys. Zeit.,
24 (1923) 185.
8b. Onsager, L.: Phys. Zeit., 27 (1926)
388.
8c. Kohlrausch, F.
and Maltby: Wiss. Abh. D. Physik. Technischen
Reichsanstalt, 3 (1900) 155; from [4b].
9. Bjerrum, N.: K. Danske Vidensk.
Selsk., 1926, 7; Proc. 7th Internl. Congr. Of Appl. Chem., London, 1909,
p. 55.; from [4b] and [10].
10. Robinson, R. A. and Stokes, R. H.: “Electrolyte Solutions“, Butterworths, London, 1955 & 1970.
11. Archer, D. G.: J. Phys. Chem. Ref.
Data, 20 (1991) 509; 21 (1992) 793; 28 (1999) 1.
12. Pitzer, K. S., Peiper, J. C. and Bussey, R. H.: J. Phys. Chem. Ref. Data, 13 (1984) 1; Krumgalz, R.,
Pogorelsky, R. and Pitzer, K. S.: J. Phys. Chem. Ref. Data, 25 (1996)
663.
13. Darvell, B. W. and Leung, V. W-H.:
Chemistry in Britain, 27 (1991) 29; Franks, F.: Chem. Britain, 27
(1991) 315.
14. Ohtaki, H. and Fukushima, N.: J. Solution Chem., 21 (1992) 23; Ohtaki, H.: Pure
Appl. Chem., 65 (1993) 203
IV: [15] – [62], author: R. Heyrovská:
(Full list in: http://www.jh-inst.cas.cz/~rheyrovs)
15. Dependence
of van't Hoff‘s factor, partition function, Yesin-Markov and transfer coefficients
on the partial molar volume.
157th
Meeting of Electrochemical Society, USA, St. Louis, Vol. 80-1 (1980)
Extd. Abstr. no. 526.
16. van't
Hoff's factor for non-ideality of gases and aqueous solutions; hydration
numbers from osmotic coefficients; Langmuir's formula extended for space
coverage.
159th Meeting of Electrochemical Society, USA, Minneapolis, Vol.
81-1 (1981) Extd. Abstr. no. 487.
17.
Simple inter-relations describing the concentration dependences of osmotic
pressure, degree of dissociation and equivalent conductivity of electrolyte
solutions.
165th
Meeting of Electrochemical Society, USA, Cincinnati, Vol. 84-1 (1984)
Extd. Abstr. no. 425.
(100th
Anniversary: Arrhenius‘ Ph.D. thesis in 1884.)
18. A
simple equation connecting diffusion coefficient, coefficient of viscosity,
concentration and osmotic pressure of electrolyte solutions; dynamics of
Brownian motion.
165th
Meeting of Electrochemical Society, USA, Cincinnati, Vol. 84-1 (1984)
Extd. Abstr. no. 426.
(100th
Anniversary: Arrhenius‘ Ph.D. thesis in 1884.)
19. A
unified representation of properties of dilute and concentrated solutions
without activity coefficient, further support: the linear dependence of E.M.F.
on lnpos
166th
Meeting of Electrochemical Society, USA, New Orleans, Vol. 84-2 (1984)
Extd. Abstr. no. 653.
(100th
Anniversary: Arrhenius‘ Ph.D. thesis in 1884.)
20. Concise
equations of state for gases and solutions: PVf = i*RoT and posVAfB
= i*RoT
166th
Meeting of Electrochemical Society, USA, New Orleans, Vol. 84-2 (1984)
Extd. Abstr. no. 652.
(100th
Anniversary: Arrhenius‘ Ph.D. thesis in 1884.)
21. Thermodynamic
interpretation of the E, lnp+/-
linear dependence of E.M.F. of concentration cells, without activity
coefficient.
168th
Meeting of Electrochemical Society, USA, Las Vegas, Vol. 85-2 (1985)
Extd. Abstr. no. 442.
22. Dependence
of the specific and equivalent conductivities and transport numbers on the
degree of dissociation of electrolytes.
168th
Meeting of Electrochemical Society, USA, Las Vegas, Vol. 85-2 (1985)
Extd. Abstr. no. 443.
23. Thermodynamic
interpretation of the ionic association / dissociation equilibrium in solutions
of electrolytes, without activity or osmotic coefficients.
168th
Meeting of Electrochemical Society, USA, Las Vegas, Vol. 85-2 (1985)
Extd. Abstr. no. 444.
24. Dissociation
and solvation of 1:1 strong elecrolytes in aqueous solutions.
1st
Gordon Research Conference on Physical Electrochemistry, New London, (1986).
(Poster)
25. A
simple proof for the incomplete dissociation of 1:1 strong electrolytes in
aqueous solutions: interpretation of density.
171st
Meeting of Electrochemical Society, USA, Philadelphia, Vol. 87-1 (1987)
Extd. Abstr. no. 463.
(100th
Anniversary: Arrhenius‘ important paper in Zeitschrift für physikalische
Chemie, I, 631, 1887.)
26. Hydration
numbers and degrees of dissociation of some strong acids, bases and salts in
aqueous solutions at 25oC.
171st
Meeting of Electrochemical Society, USA, Philadelphia, Vol. 87-1 (1987)
Extd. Abstr. no. 472.
(100th
Anniversary: Arrhenius‘ important paper in Zeitschrift für physikalische
Chemie, I, 631, 1887.)
27. Physical
Chemistry of solutions without activity coefficients: solvation and incomplete
dissociation of strong electrolytes.
8th
International Symposium on Solute-Solute-Solvent Interactions, Regensburg,
Germany (1987), Extd. Abstr. no. L.1.19.
(100th
Anniversary: Arrhenius‘ important paper in Zeitschrift für physikalische
Chemie, I, 631, 1887.)
28. Dependence
of e.m.f. of concentration cells on actual concentrations of ions, and `true
pH'.
8th
International Symposium on Solute-Solute-Solvent Interactions, Regensburg,
Germany (1987),
(poster) Extd. Abstr. no. P.2.23.
(100th
Anniversary: Arrhenius‘ important paper in Zeitschrift für physikalische
Chemie, I, 631, 1887.)
29. Quantitative
interpretation of properties of aqueous solutions on the basis of hydration and
incomplete dissociation of electrolytes.
172nd
Meeting of Electrochemical Society, USA, Hawaii, Vol. 87-2 (1987) Extd.
Abstr. no. 1454.
(100th
Anniversary: Arrhenius‘ important paper in Zeitschrift für physikalische
Chemie, I, 631, 1887.)
30. Quantitative
interpretation of properties of aqueous solutions in terms of hydration and `true
ionic concentrations'.
International
Symposium on Molecular and Dynamic Approaches to Electrolyte Solutions, Tokyo (1988),
Extd. Abstr. p. 44.
31. Interpretation
of properties of aqueous electrolyte solutions in terms of hydration and
incomplete dissociation.
Collection
of Czechoslovak Chemical Communications, 53 (1988) 686.
32. A
re-appraisal of Arrhenius' theory of partial dissociation of electrolytes.*
3rd
Chemical Congress of North America and 195th Meeting of the American Chemical
Society, Toronto, (1988):
In Book: Chapter 6, Same title*,"Electrochemistry,
Past and Present", American Chemical Society Symposium Series 390,
Editors: J.T. Stock and M.V. Orna, American Chemical Society Publications,
Washington DC, (1989).
33. A
re-appraisal of Arrhenius' theory of partial dissociation of electrolytes.
2nd
Gordon Research Conference on Physical Electrochemistry, New London, (1988)
(Invited Poster)
34. Degrees
of dissociation and hydration numbers of six tetra alkyl ammonium halides and
nineteen 2:1 strong electrolytes in aqueous solutions at 25oC.
Collection
of Czechoslovak Chemical Communications, 54 (1989) 1227.
35. Effective
radii of alkali halide ions in aqueous solutions, crystals and in the gas
phase, and the interpretation of Stokes ionic radii.
Chemical
Physics Letters, 163 (1989) 207.
36. Interpretation
of A) solution properties in terms of solvation and incomplete dissociation and
B) Stokes ionic radii in terms of ion-solvent interactions.
Proceedings
II, J.H. Centennial Congress on Polarography and 41st Meeting of the
International Society of Electrochemistry, Prague, 1990. (Poster) Extd.
Abstr. Fr-112.
37. Ionic
concentration outlives ionic strength.
Chemistry
in Britain, 27 (1991) 1114.
38. Degrees
of dissociation and hydration numbers of twenty six strong electrolytes in
aqueous solutions at 25oC.
Collection
of Czechoslovak Chemical Communications, 57 (1992) 2209.
39. A:
Incomplete dissociation of NaCl and 99 other strong electrolytes in a `sea' of
water; B: "Ionic radii" and the mystery of "Stokes ionic
radii".
"Futures in Marine Chemistry, XIIth International
Symposium", May 1993, Brijuni, Croatia. (2 posters in English)
40. Physical
electrochemistry of strong electrolyte solutions based on partial dissociation
and hydration.
187th
Mtg of the Electrochemical Society, USA, Reno, USA, Vol. 95-1 (1995)
Extd. Abstr. no.662
(100th
Anniversary: ARRHENIUS was appointed as Professor of Physics in
Stockholms Högskola in 1895.)
41. YES
ARRHENIUS, alkali halides are incompletely dissociated at all concentrations in
water.
The
Autumn Meeting of The Royal Society of Chemistry, Sheffield, UK, Sept. (1995).
(Poster)
(100th
Anniversary: ARRHENIUS was appointed as Professor of Physics in
Stockholms Högskola in 1895.)
42. Physical
chemistry of the kitchen salt in aqueous solutions.
The
Autumn Meeting of The Royal Society of Chemistry, Faraday Symposium on "Ions
in Solution", Sheffield, UK, Sept. (1995). (Lecture)
(100th
Anniversary: ARRHENIUS was appointed as Professor of Physics in
Stockholms Högskola in 1895.)
43. Physical
electrochemistry of strong electrolytes based on partial dissociation and
hydration: quantitative interpretation of the thermodynamic properties of
NaCl(aq) from "zero to saturation".
Journal
of Electrochemical Society, 143 (1996) 1789; (with Tables of
data). Text in: http://www.jh-inst.cas.cz/~rheyrovs
(100th
Anniversary: ARRHENIUS at the top of the world in Spitzbergen with the Polar explorer Andree, 1896!)
44. Partial
dissociation and hydration of strong acids and the significance of
"pH".
Abstract,
p. 13, Moderni Elektroanalyticke Metody XVI, Harrachov, Czech Rep., May
14-16, 1996. (in English)
(100th
Anniversary: ARRHENIUS at the top of the world in Spitzbergen with the Polar explorer Andree, 1896!)
45. Degrees
of dissociation and hydration numbers of alkali halides in aqueous solutions at
25oC (some up to saturation)
Croatica
Chemica Acta, 70 (1997) 39. (with Tables of data)
46. Equations
for densities and dissociation constant of NaCl(aq) at 25oC from
"zero to saturation" based on partial dissociation
Journal
of Electrochemical Society, 144 (1997) 2380.
47. Bjerrum's
theory for ionic association in NaCl(aq) at 25oC from "zero to
saturation".
Abstract
p.12. International Conference on Inorganic Environmental Analysis &
Quality Assurance, Pardubice, Czech Rep., Sept. 2-5, 1997. (Poster in
English)
48. Physical
electrochemistry of solutions of strong electrolytes (partial dissociation and
hydration from "zero to saturation")
Chemicke Listy, 92 (1998) 157. (A
review in English), with Tables of data, also in:
http://www.jh-inst.cas.cz/~rheyrovs
49. Notes
on hydration theory
In
Book (in Chapter 11): "Ionic
Equilibrium", Ed.: J. N. Butler (John Wiley and Sons, New York, (1998).
50. No
kidding! Strong electrolytes are only partially dissociated in aqueous
solutions at all concentrations as Arrhenius supposed!
216th
National Meeting of the American Chemical Society, Boston, Aug. 1998,
short abstract no. 82. (Poster)
51. A
remark on Bjerrum's theory of ionic association: partial dissociation of
NaCl(aq) from "zero to saturation" at 25oC.
Journal
of Molecular Liquids 81 (1999) 83; (with a Table of data).
52. Festina
Lente (Hurry Slowly): The development of the theory of electrolytes.
Chemical
Heritage Magazine, March 1999. (Abstract of talk by R. Heyrovská)
53. Volumes
of ions, ionpairs and electrostriction of alkali halides in aqueous solutions
at 25oC
217th
National Meeting of the American Chemical Society, Anaheim, March 1999,
Abstract no. 61.
Marine
Chemistry, 70 (2000) 49. (Proceedings, Dedicated to Frank J. Millero on
the occasion of his 60th birthday); (with Tables of data)
54. Degrees
of dissociation and hydration numbers of M2SO4 (M = H,
Li, Na, K, Rb, Cs and NH4) in aqueous solutions at 25oC.
1999
Joint International Meeting (196th Meeting of The Electrochemical Society, USA,
1999 Fall Meeting of The Electrochemical Society of Japan with technical
cosponsorship of The Japan Society of Applied Physics), Honolulu, Hawaii,
October 1999, Extd. Abstr. of the ECS, Vol. 99 –2, no. 2041, 1999; (with
a Table of data).
55. Thermodynamic
significance of transfer coefficients. (Involves partial
dissociation)
2nd
Workshop of Physical Chemists and Electrochemists: "Physical chemistry and
Electrochemistry at the end of the second Millenium", Masaryk University,
Brno, February 2000. Book of Abstracts, page 11. (in English)
56. The
Theory of Electrolytes.
Chemical
Heritage 18 (2000) 29; (by M. V. Orna, abstract of talk by R.
Heyrovska')
57. Sorry
Lewis, Bancroft was right: the concentration/activity controversy and the
survival of the Journal of Physical Chemistry.
219th
Meeting of The American Chemical Society, San Francisco, March 2000,
Abstr. no. 37.
58. J.
Heyrovsky's data in 1923 on the deposition potentials of alkali metal cations
interpreted here in terms of partial dissociation and hydration.
J.
Heyrovsky Memorial Symposium on Advances in Polarography and Related Methods,
Prague, Czech Republic, August/September 2000. Extended abstract, p. 36
(in English).
59. Recent
success of the theory of partial dissociation and hydration of electrolytes (A
tribute to van't Hoff and Arrhenius on the occasion of the Nobel Centennial,
2001)
Prague
- Dresden Electrochemical Seminar, Jetrichovice, Czech Republic, December 2001.
Book of Abstracts, p. 15 (in English)
60. E.m.f.
of cells: simple dependence on hydration, partial dissociation and transfer
coefficient (not on activity coefficients and extended Debye-Huckel equations!)
3rd
Workshop of Physical Chemists and Electrochemists, Masaryk University, Brno,
February 2002, Book of abstracts, page 24. (In English)
61. Comments
on the Pitzer equations formulated on the assumption of complete dissociation
of strong electrolytes.
Journal
of Physical and Chemical Reference Data, Volume 29, No. 4, 2000
(assigned volume and number after accepting and proof-reading, but
the article was not published!)
62. A
Concise Equation of State for Aqueous Solutions of Electrolytes Incorporating
Thermodynamic Laws and Entropy.
(Invited paper) Special Issue of ENTROPY, December 1, 2003.
(100th Anniversary: ARRHENIUS was awarded the Nobel Prize
in 1903!)
Fig. 1a: Dilute Solutions
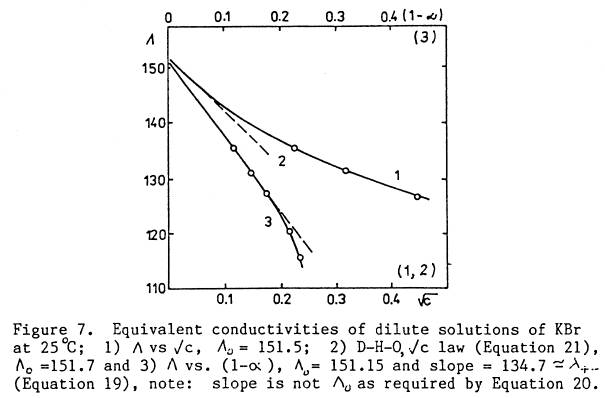
Fig. 1b: 0.05 to 3.75M
From:
R. Heyrovská, Chapter 6 "Electrochemistry, Past and Present",
ACS Symp. Series 390, Eds: J.T. Stock and M.V. Orna, ACS Publications,
Washington DC, (1989).
Fig. 2. KBr, “0 to 3.75M”
From:
R. Heyrovská, Chapter 6 "Electrochemistry, Past and Present",
ACS Symp. Series 390, Eds: J.T. Stock and M.V. Orna, ACS Publications,
Washington DC, (1989).
Fig. 3. NaCl(aq) at 250C: “0 to saturation (6.14m)”
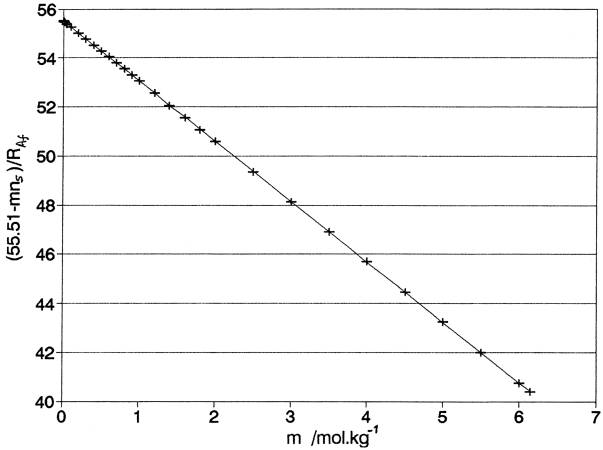
Slope = - nb = -2.457 (S.E., 0.001); intercept =
55.51 (S.E., 0.01)
From: R. Heyrovská,
Journal of Electrochemical Society, 143 (1996) 1789.
Fig. 4. NaCl(aq), “0 to saturation”
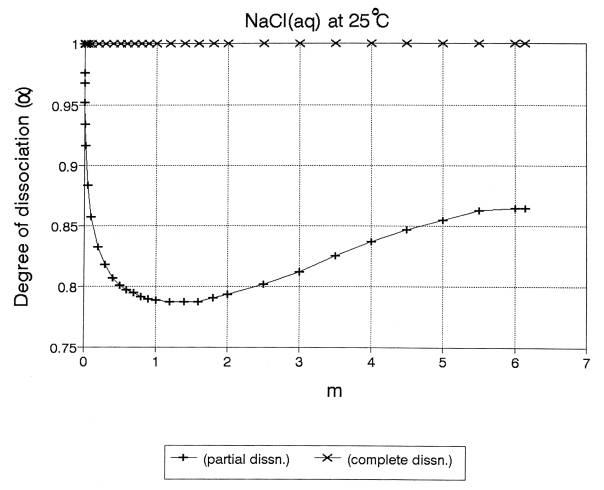
From: R. Heyrovská, Journal of Electrochemical Society, 143 (1996)
1789.
Table 1. NaCl(aq), a at various m
Degrees
of dissociation (a) at various molalities (m)
(6.144m: satd. soln.) for NaCl (aq) at 25oC,
RAf (Eq. 7)
and the
comparison of f (Eq. 4) with f.14
--------------------------------------------------------------------------
m f14
f(Eq.4) RAf a(Eq.6) a(Eq.4)
---------------------------------------------------------------------------
0,000 1,000
1,000 1,00000 1,000 1,000
0,001 0,988
0,988 0,99998 0,976 0,976
0,002 0,984
0,984 0,99996 0,968 0,968
0,005 0,976
0,976 0,99991 0,952 0,952
0,010 0,968
0,968 0,99983 0,935 0,935
0,020 0,959
0,959 0,99965 0,916 0,916
0,050 0,944
0,944 0,99915 0,884 0,884
0,100 0,933
0,933 0,99832 0,858 0,858
0,200 0,924
0,924 0,99667 0,832 0,832
0,300 0,921
0,921 0,99503 0,818 0,818
0,400 0,920
0,920 0,99339 0,808 0,807
0,500 0,921
0,921 0,99173 0,801 0,801
0,600 0,923
0,923 0,99006 0,797 0,797
0,700 0,926
0,926 0,98837 0,795 0,795
0,800 0,929
0,929 0,98667 0,792 0,792
0,900 0,932
0,932 0,98497 0,790 0,790
1,000 0,936
0,936 0,98323 0,789 0,789
1,200 0,944
0,944 0,97973 0,788 0,788
1,400 0,953
0,953 0,97616 0,788 0,788
1,600 0,962
0,962 0,97253 0,787 0,788
1,800 0,973
0,973 0,96878 0,791 0,791
2,000 0,984
0,984 0,96497 0,793 0,794
2,500 1,013
1,013 0,95507 0,801 0,802
3,000 1,045
1,045 0,94459 0,812 0,812
3,500 1,080
1,080 0,93345 0,826 0,825
4,000 1,116
1,116 0,92174 0,837 0,837
4,500 1,153
1,153 0,90944 0,847 0,847
5,000 1,191
1,192 0,89656 0,856 0,855
5,500 1,231
1,231 0,88298 0,863 0,863
6,000 1,270
1,270 0,86900 0,865 0,865
6,144 1,281
1,280 0,86491 0,864 0,865
From: R. Heyrovská, Journal of Electrochemical Society, 143 (1996)
1789.
Table 2. NaCl(aq), d and V vs dcal
and Vcal at various m
The
densities (d) (g/cm3),18 molal volumes (V) (cm3),
degrees
of dissociation (a), and ionic
molalities (am) of NaCl(aq) at 25oC. Vcal
and dcal are the calculated values. Km
values are as per Eq. 8. d for the
saturated solution is from Ref. 25.
----------------------------------------------------------------------------------
m
d
V a am Vcal d - dcal Km
-----------------------------------------------------------------------------------
0.000 0.99709
1002.92 1.000 0.000 1002.86
-0.00006 ------
0.100 1.00117
1004.67 0.858 0.086 1004.63
-0.00003 0.52
0.250 1.00722
1007.34 0.825 0.206
1007.38 0.00005 0.97
0.500 1.01710
1011.92 0.801 0.401
1012.03 0.00012 1.61
0.750 1.02676
1016.63 0.793 0.595 1016.69
0.00006 2.28
1.000 1.03623
1021.44 0.789 0.789 1021.34
-0.00010 2.95
2.000 1.07228
1041.60 0.794 1.587
1041.64 0.00004 6.10
3.000 1.10577
1062.91 0.812 2.436 1062.65
-0.00027 10.52
4.000 1.13705 1085.06
0.837 3.348 1085.21 0.00015
17.19
5.000 1.16644
1107.83 0.855 4.275
1108.14 0.00033 25.21
6.000 1.19423
1130.99 0.865 5.190 1130.78
-0.00022 33.25
6.144 1.1978
1134.64 0.865 5.315 1133.86
-0.00082 34.05
From: R. Heyrovská, Journal of Electrochemical Society, 143 (1996)
1789.
Fig. 5. f, g±
(complete
dissocn.) & a (partial dissocn.)
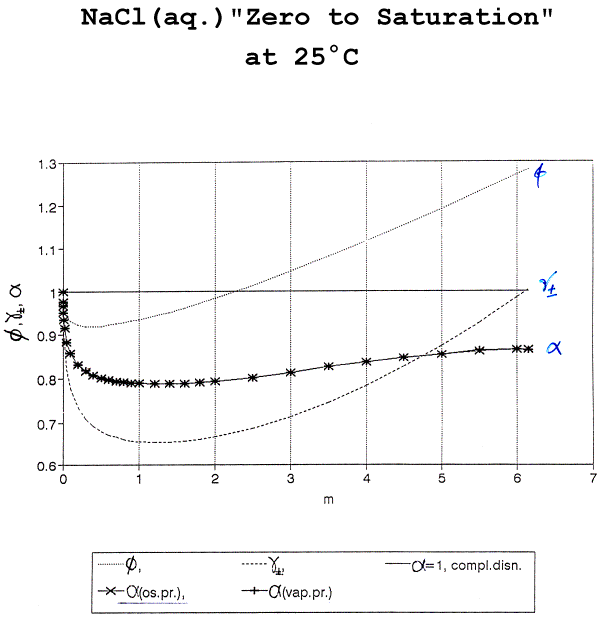
From: R. Heyrovská, Chemicke Listy, 92 (1998) 157.
Fig. 6. DE vs. ln (am/nAf)
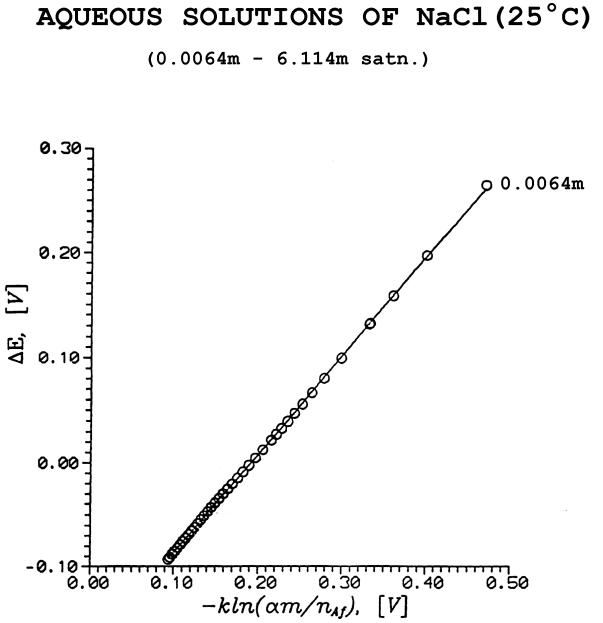
From: R. Heyrovská, first graph obtained in April 1994.
Fig. 7. Vm (c.d.) vs Vm
(p.d.) “0 to
saturation”
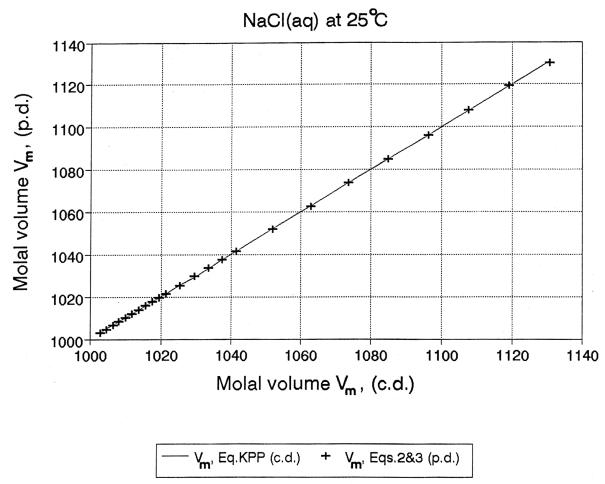
X-axis: Vm (c.d.) calculated from the parameters of Krumgalz,
Pogorelsky and Pitzer equation, JPCRD, 1996.
Y-axis: Vm (p.d.) calculated from volumes of ions, ion pairs, water and
electrostriction.
R. Heyrovská, Journal of Electrochemical Society, 143 (1996) 1789; 144 (1997) 2380.
Fig. 8. Surface vs bulk hydration numbers:
(ns vs nb)
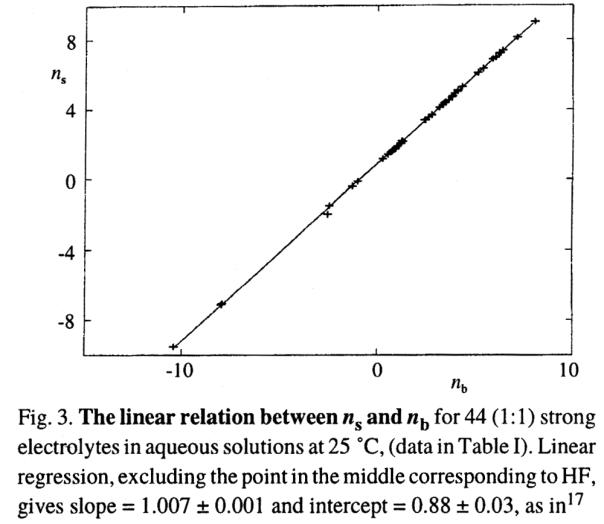
From: R. Heyrovská, Chemicke Listy, 92 (1998) 157.
Table 3
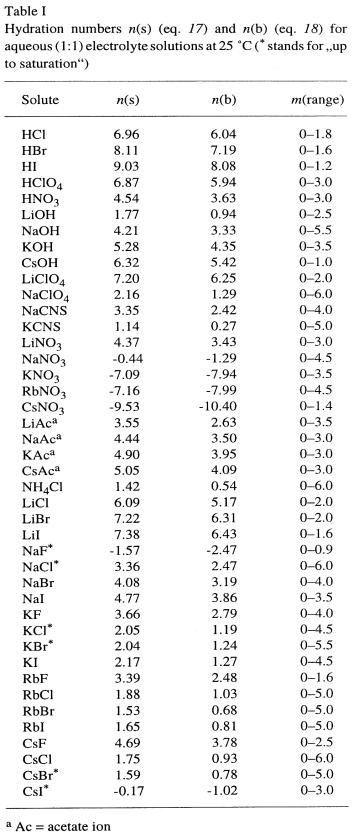
From: R. Heyrovská, Chemicke Listy, 92 (1998) 157.
Fig. 9. NaCl(aq) at 250C, “0 to saturation”
(Distance of closest
approach, a vs m)
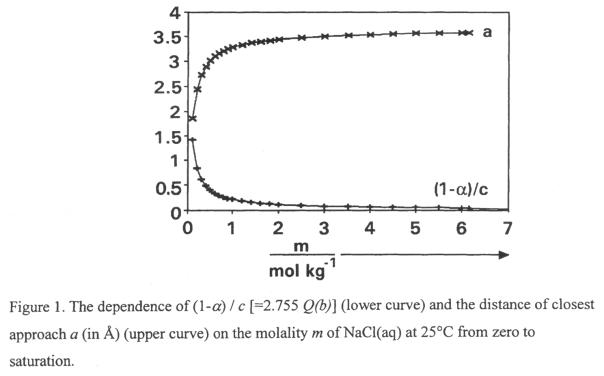
From: R. Heyrovská, Journal of Molecular Liquids 81 (1999) 83.
Photos, I & II
I: From: E. Crawford, “Arrhenius: Ionic
theory to the greenhouse effect”, 1996


II. Photo of the same place in 1967 (by R.
Heyrovská)