







 |
|
JH-suite
© Alan Liška, Veronika
Růžičková, Jiří Ludvík, 2017-2019
JH-suite (named according
to Jaroslav Heyrovský) is a set of small codes compiled for both Windows
and Android platforms enabling fast thermodynamic estimations (prediction
of equilibrium constants, calculation of fusion enthalpy, atomic charges
etc.), prevealingly based on empirical models published in literature
during several past decades. Although the today´s routines in the field
rely mainly on quantum chemical modelling, the older empirical
procedures are still interesting due to (a) the concepts themselves (their
linkage to the more recent theories), and especially, (b) almost zero
computational demands. The program suite (developed in cooperation with
Institute of Geochemistry, Mineralogy and Natural Resources, Faculty of
Science, Charles University in Prague)
is offered as freeware with no garancy. However, any report on bugs,
improvements ideas etc. will be welcomed (please
send an e-mail to alan.liska@jh-inst.cas.cz).
Before use, please read the
corresponding manual packed inside each zip file.
Windows version is
compatible with any modern version of Windows (XP and later). On the
other hand, it won´t run under DOS, even if using a 32bit extender.
Android version
requires installation of X11-BASIC. The currently offered binaries work with version 1.25-48
and up. The installation directory in the Android device must be
/mnt/sdcard/jh/[name of particular
program]/, otherwise the program will
crash. The easiest way of execution of any program (with .b extension)
is from a suitable file explorer (e.g.
Total Commander). The
input / output / parameter files can be handled by any text editor (e.g.
920 Text Editor).
Structure of JH-suite
Currently, JH-suite
consists of 41 individual codes enabling empirical estimations in the
field of both inorganic and organic chemistry, from analytical
equilibrium constants through rate coefficients to molecular properties
and organic compounds characteristics.
The program is equipped by
a simple GUI, which enables to choose the appropriate code for
performing the desired calculation. All the project is based on
X11-Basic which was chosen as a programming workbench in order to
facilitate the code to run on both Windows and Android platforms. To
display properly the individual input, database, parameter and output
files, it is highly recommended to use
Notepad++
(Windows) /
920 Text Editor (Android).
| |
Inorganic |
Organic |
General |
| Acid-Base |
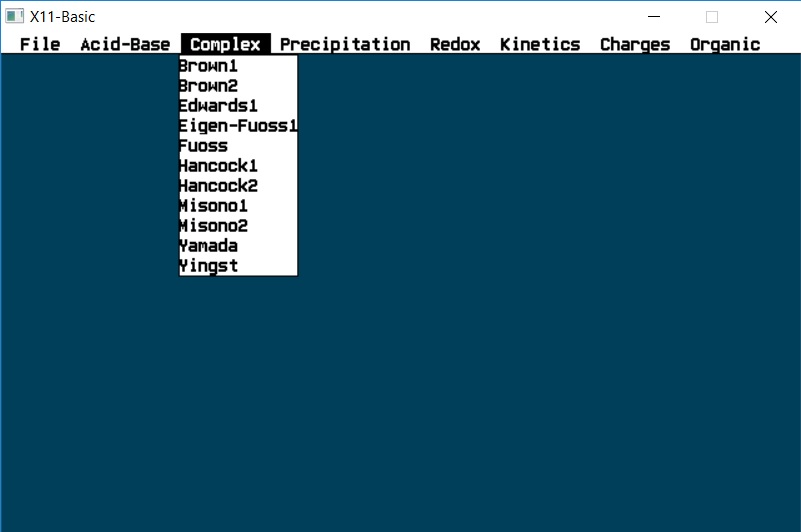
Complex
formation |
Precipitation |
Redox |
Thermodynamics
(equilibria) |
Bayless |
Brown1 |
Brown3 |
Brown4 |
Banks-Burnop |
Pauling2 |
| Bayless-Ricci |
Brown2 |
Clifford1 |
|
Brown-Stein |
PCM |
| Bell |
Edwards1 |
Clifford2 |
|
Dick |
Sanderson |
| Pauling1 |
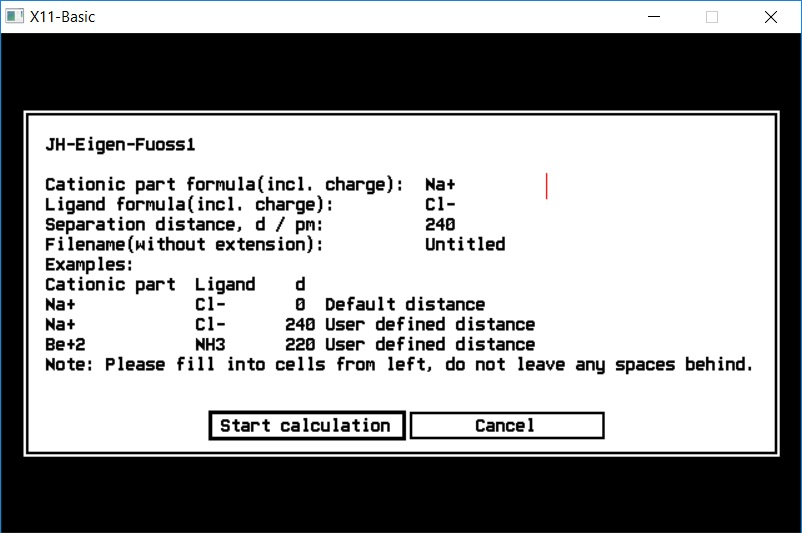
Eigen-Fuoss1 |
|
|
Hammett2 |
|
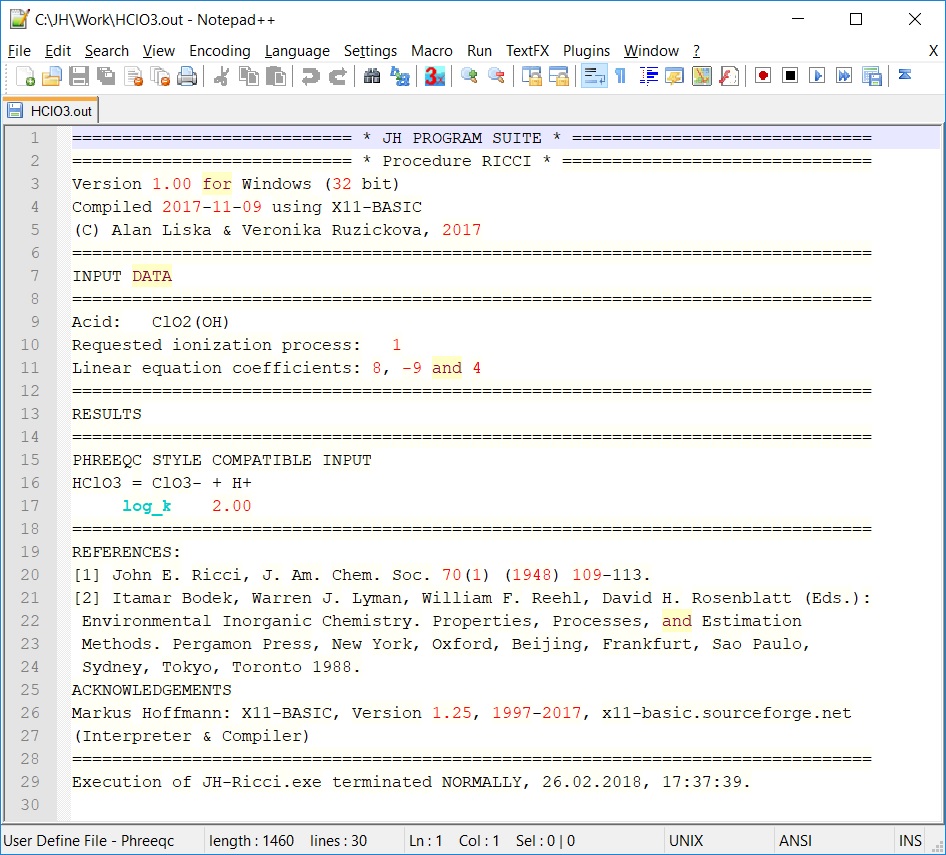
| Ricci |
Fuoss |
|
|
Joback |
|
| |
Hancock1 |
|
|
Mavrovouniotis |
|
| |
Hancock2 |
|
|
Pedley |
|
| |
Misono1 |
|
|
Polymer |
|
| |
Misono2 |
|
|
Simamora |
|
| |
Yamada |
|
|
Zuman1 |
|
| |
Yingst |
|
|
Zuman2 |
|
| |
|
|
|
Zuman3 |
|
| |
|
|
|
Zuman4 |
|
| Kinetics |
Zhou |
Edwards2 |
|
Marcus |
Hammett2 |
|
| |
Eigen-Fuoss2 |
|
|
|
|
Brief description of components:
| Code |
Description |
| Banks-Burnop |
boiling points of organic compounds |
| Bayless |
protonization constants for inorganic acids |
| Bayless-Ricci |
protonization constants for inorganic acids |
| Bell |
protonization constants for inorganic oxo acids |
| Brown1 |
complex formation equilibria, parametrized hard & soft acid-base
principle, inorganic ligands |
| Brown2 |
complex formation equilibria, parametrized hard & soft acid-base
principle, organic ligands: amines, carboxylic acids and amino
acids |
| Brown3 |
precipitation equilibria, parametrized hard & soft acid-base
principle, inorganic species |
| Brown4 |
redox
equilibria, parametrized hard & soft acid-base principle,
inorganic species |
| Brown-Stein |
melting and boiling
points of organic compounds |
| Clifford1 |
precipitation equilibria,
electronegativity calculation scheme |
| Clifford2 |
precipitation equilibria,
electronegativity calculation scheme |
| Dick |
equilibria among amino
acids and peptides |
| Edwards1 |
complex formation
equilibria, parametrized hard & soft acid-base principle |
| Edwards2 |
rate constants of
nucleophilic substitution on Pt(II)-centre |
| Eigen-Fuoss1 |
complex formation equilibria, outer sphere aducts |
| Eigen-Fuoss2 |
rate constants of outer
sphere nucleophilic substitutions in complexes |
| Fuoss |
complex formation equilibria, ion pairs |
| Hammett1 |
organic acids
protonization constants |
| Hammett2 |
selected organic
reactions rate coefficients |
| Hancock1 |
complex formation equilibria, parametrized hard & soft acid-base
principle |
| Hancock2 |
complex formation equilibria, parametrized hard & soft acid-base
principle, generalized for both small and large donor atoms |
| Joback |
basic organic
thermodynamic estimations |
| Marcus |
redox potentials of
elementary reactions |
| Mavrovouniotis |
standard Gibbs energy of
aqueous organic solutes |
| Misono1 |
complex formation
equilibria, parametrized hard & soft acid-base principle |
| Misono2 |
complex formation
equilibria, parametrized hard & soft acid-base principle |
| Pauling1 |
protonization constants of inorganic oxo acids, empirical rules |
| Pauling2 |
standard enthalpy of
fussion of any compound in its standard state |
| Pedley |
standard enthalpy of
fussion of organic compounds |
| PCM |
Partial Charge Model, calculation of atomic charges in any
species - molecules, ions - based on electronegativity
equalization principle |
| Polymer |
thermodynamic,
spectroscopic, mechanical and other properties of organic
compounds and polymers from group contributions |
| Ricci |
protonization constants for inorganic oxo acids, empirical rules |
| Sanderson |
thermodynamic properties and charge distribution based on one of
the broadest empirical calculation scheme |
| Simamora |
melting and boiling
points of organic compounds where no hydrogen-bonds are employed |
| Yamada |
complex formation
equilibria, parametrized hard & soft acid-base principle |
| Yingst |
complex formation equilibria, parametrized hard & soft acid-base
principle |
| Zhou |
rate constants of e.g.
acid-base reactions and other diffussion controlled processes |
| Zuman1 |
polarographic half-wave
potentials of aromatic compounds |
| Zuman2 |
polarographic half-wave
potentials of heterocyclic compounds |
| Zuman3 |
polarographic half-wave
potentials of aliphatic compounds |
| Zuman4 |
polarographic half-wave
potentials of heterocyclic compounds |
For more information, please read the
manuals.
Distribution

|





